Cause of chemical shifts (ll)
· One is Inductive effect which was stated earlier (in
previous post).
· Hybridisation of C atom:
This is
somehow related to inductive effect. There are generally three types of
hybridisation of carbon observed in organic compounds( sp3 sp2 and sp). We know
that more the ‘s’ character more will be the deshielding (more ‘s’ character
shows availability of electron cloud towards more to the nucleus) and more will
be chemical shift.
According to
above , the order of chemical shift will be:
sp > sp2 > sp3
but this is
not actual order , the order is :
sp2 > sp3 > sp
This trend
is explained by another term called Magnetic Anisotropy.
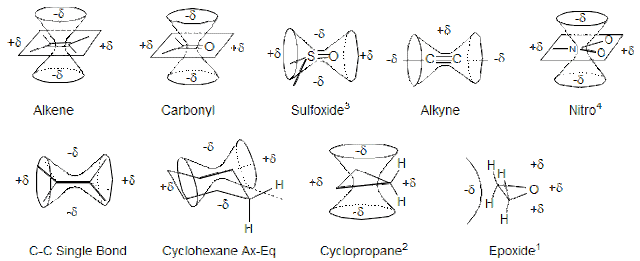
· Magnetic Anisotropy:
The word
anisotropy means non spherical environment. For example let us take a C-C
single bonded carbon atom. When we see the electron cloud around this C-C bond
we found that this is not spherical which create non uniform magnetic field.
Due to which
one part of a molecule becomes shielded and another become deshielded. The
nucleus which comes under electron cloud region are said to shielded nuclie and
another one is deshielded nuclie.
In the above
figure the double cone shaped region is shielded region.
Acetaldehyde ( δ (ppm) ~9.7)
Ethyne (δ (ppm) ~ 2.5)
- · Mesomeric effect or Resonance effect:In Resonance effect different number of resonating structure obtained and in these structures some atom accumulate positive charge and some accumulate negative charge. This accumulation of charge causes in shielding and deshielding of proton attached to them. Ex:-
· Stearic Effect or Vander Wall’s Deshielding :
Generally it
is observed where bulky groups were present around Hydrogen nuclei. The electron
cloud of bulky group repel each other and hence result in the deshielding of
Hydrogen nuclei.
- Hydrogen bonding:







Comments
Post a Comment