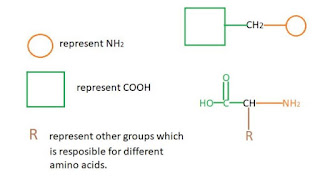
Cont... 3+3+2+2 rule
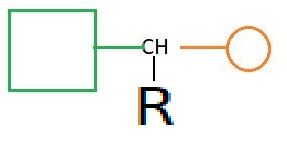
Now this is the time to learn another 10 amino acids. THIS TIME ONLY BY CHANGING 'R' WE GET ALL OTHER AMINO ACIDS. So lets start: AROMATIC REMEMBER ; All aromatic amino acid's name starts with sounds "ty" except phenylalanine which has itself a word "phenyl" which indicates aromatc amino acid. 1. PHENYLALANINE :- It is a phenyl derivative of ALANINE. It has R = -CH 2 C 6 H 5 2. TYROSINE :- It is a phenol derivative of ALANINE it has R = -CH 2 C 6 H 4 OH 3. TRYPTOPHANE :- It is a indole derivative of Glycine . It has R as Indole group BASIC REMEMBER ; All basic amino acids have 3 carbon as side chain except LYSINE whose name suggest that he is LIER ( LYSINE has 4 carbon as side chain). 1. HISTIDINE :- It has R = imidazole ring as a side chain. 2. LYSINE :- As mentioned above that this molecule is a lie...