conversion of glucose's open chain form to chair form
How to understand the chair form of glucose
Generally for us it is easier to understand the structure in 2D. But if we have to know about the configuration (arrangement of substituents) of a molecule in 3D which is already represented on paper in 2D then we have to visualize that molecule in mind in 3D . This visualization helps to understand the correct structure of molecule. So let's go forward for the same.
Glucose which has the molecular formula= C6H12O6 . It is aldohexose sugar which contains an aldehyde and 5 -OH groups attached to 5 different carbon.Its linear structure is given below
 |
| D-Glucose |
1- Assign numbers to each carbon according to PRIORITY
2 Rotate it in 90° clockwise direction
3 make cyclic bond with CHO group to carbon 5 and at carbon 1 new -H and -OH bond is formed. Here remember that while making bond with C1 to oxygen of C5 , new H and OH bond at C1 carbon are formed . If we put OH downward then it is alpha-D glucose otherwise it is beta-D glucose.
alpha-D glucose
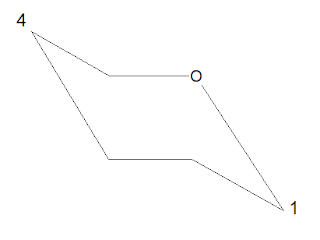
4 Stretch the above cyclic structure in a way as bath tub by making base as 2,3 carbon
and carbon1 and carbon4 in upward direction
 |
6. completing this we get HAWORTH STRUCTURE
Upto this we get 2D Haworth structure of 𝛃-D glucose
Conversion of Haworth to chair form:-
1. Stretch C1 downward and C4 upward (this can be reversed C1 upward and C4 downward both give correct structure) but here remember only this way.
2 now assign H and OH groups to each carbon
3 This is the final chair form of alpha D-glucose







Comments
Post a Comment